Learning Objectives
- Explain the roles of subscripts and coefficients in chemical equations.
- Balance a chemical equation when given the unbalance equation.
- Explain the role of the Law of Conservation of Mass in a chemical reaction.
Even though chemical compounds are
broken up and new compounds are formed during a chemical reaction, atoms
in the reactants do not disappear nor do new atoms appear to form the
products. In chemical reactions, atoms are never created or destroyed.
The same atoms that were present in the reactants are present in the
products - they are merely reorganized into different arrangements. In a
complete chemical equation, the two sides of the equation must be
present on the reactant and the product sides of the equation.
Coefficients and Subscripts
There are two types of numbers that
appear in chemical equations. There are subscripts, which are part of
the chemical formulas of the reactants and products and there are
coefficients that are placed in front of the formulas to indicate how
many molecules of that substance is used or produced.
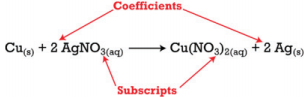
The subscripts are part
of the formulas and once the formulas for the reactants and products
are determined, the subscripts may not be changed. The coefficients
indicate the number of each substance involved in the reaction and may
be changed in order to balance the equation. The equation above
indicates that one mole of solid copper is reacting with two moles of
aqueous silver nitrate to produce one mole of aqueous copper (II)
nitrate and two atoms of solid silver.
Balancing a Chemical Equation
Because the identities of the reactants
and products are fixed, the equation cannot be balanced by changing the
subscripts of the reactants or the products. To do so would change the
chemical identity of the species being described, as illustrated in
Figure
.
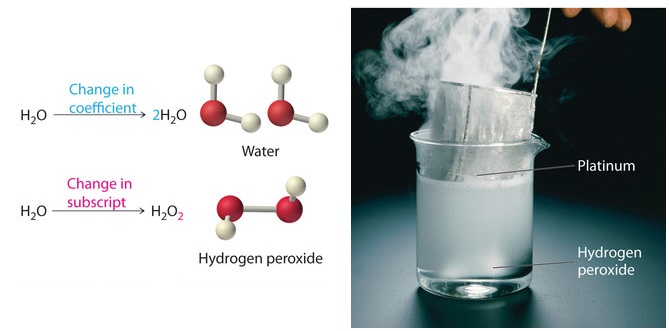
The simplest and most generally useful
method for balancing chemical equations is “inspection,” better known as
trial and error. The following is an efficient approach to balancing a
chemical equation using this method.
Steps in Balancing a Chemical Equation
- Identify the most complex substance.
- Beginning with that substance, choose an element(s) that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element(s) on both sides.
- Balance polyatomic ions (if present on both sides of the chemical equation) as a unit.
- Balance the remaining atoms, usually ending with the least complex substance and using fractional coefficients if necessary. If a fractional coefficient has been used, multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients.
- Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced.
Example
: Combustion of Heptane
Balancing the chemical Equation for the combustion of Heptane ()
| 1. Identify the most complex substance. | The most complex substance is the one with the largest number of different atoms, which is |
| . We will assume initially that the final balanced chemical equation contains 1 molecule or formula unit of this substance. | |
| 2. Adjust the coefficients. | a. Because one molecule of n-heptane contains 7 carbon atoms, we need 7 CO2 molecules, each of which contains 1 carbon atom, on the right side: |
| Reactants | Element/Polyatomic Ion | Products |
|---|---|---|
| 7 | C | 7 |
|
|||||||||
| 3. Balance polyatomic ions as a unit. | There are no polyatomic ions to be considered in this reaction. | ||||||||
| 4. Balance the remaining atoms. | The carbon and hydrogen atoms are now balanced, but we
have 22 oxygen atoms on the right side and only 2 oxygen atoms on the
left. We can balance the oxygen atoms by adjusting the coefficient in
front of the least complex substance, O2, on the reactant side: |
|
||||||||||||
| 5. Check your work. | The equation is now balanced, and there are no fractional coefficients: there are 7 carbon atoms, 16 hydrogen atoms, and 22 oxygen atoms on each side. Always check to be sure that a chemical equation is balanced. |
Example
: Combustion of Isooctane
Combustion of Isooctane ()
The assumption that the final balanced
chemical equation contains only one molecule or formula unit of the most
complex substance is not always valid, but it is a good place to start.
The combustion of any hydrocarbon with oxygen produces carbon dioxide
and water.
| 1. Identify the most complex substance. | The most complex substance is the one with the largest number of different atoms, which is |
| . We will assume initially that the final balanced chemical equation contains 1 molecule or formula unit of this substance. | |
| 2. Adjust the coefficients. | a. The first element that appears only once in the
reactants is carbon: 8 carbon atoms in isooctane means that there must
be 8 CO2 molecules in the products: |
| Reactants | Element/Polyatomic Ion | Products |
|---|---|---|
| 8 | C | 8 |
|
|||||||||
| 3. Balance polyatomic ions as a unit. | There are no polyatomic ions to be considered in this reaction. | ||||||||
| 4. Balance the remaining atoms. |
The carbon and hydrogen atoms
are now balanced, but we have 25 oxygen atoms on the right side and only
2 oxygen atoms on the left. We can balance the least complex substance,
O2, but because there are 2 oxygen atoms per O2 molecule, we must use a fractional coefficient (
|
) to balance the oxygen atoms:
| Reactants | Element/Polyatomic Ion | Products |
|---|---|---|
| 8 | C | 8 |
| 18 | H | 18 |
| 25 | O | 25 |
The
equation is now balanced, but we usually write equations with
whole-number coefficients. We can eliminate the fractional coefficient
by multiplying all coefficients on both sides of the chemical equation
by 2:
| 5. Check your work. | The balanced chemical equation has 16 carbon atoms, 36 hydrogen atoms, and 50 oxygen atoms on each side.
Balancing equations requires
some practice on your part as well as some common sense. If you find
yourself using very large coefficients or if you have spent several
minutes without success, go back and make sure that you have written the
formulas of the reactants and products correctly.
|
Example
: Precipitation of Lead (II) Chloride
Aqueous solutions of lead (II) nitrate and sodium chloride are mixed.
The products of the reaction are an aqueous solution of sodium nitrate
and a solid precipitate of lead (II) chloride. Write the balanced
chemical equation for this reaction.SOLUTION
| 1. Identify the most complex substance. |
The most complex substance is lead (II) chloride.
|
| 2. Adjust the coefficients. | There are twice as many chloride ions in the reactants
than in the products. Place a 2 in front of the NaCl in order to balance
the chloride ions. |
|
||||||||||||
| 3. Balance polyatomic ions as a unit. | The nitrate ions are still unbalanced. Place a 2 in front of the NaNO3. The result is: |
|
|||||||||||||||
| 4. Balance the remaining atoms. | There is no need to balance the remaining atoms because they are already balanced. | ||||||||||||||
| 5. Check your work. |
|
Exercise
Is each chemical equation balanced?
- Answer a
- Answer b
- Answer c
Exercise
- Answer a
- Answer b
- Answer c
Summary
- To be useful, chemical equations must always be balanced. Balanced chemical equations have the same number and type of each atom on both sides of the equation.
- The coefficients in a balanced equation must be the simplest whole number ratio. Mass is always conserved in chemical reactions.




0 Comments