Conformers are abserved due to free rotation arround sigma bond. There are infinite Conformation of a compound, and specific Conformation of a compound known as conformers.
> Conformers are stable Conformation of a compound.
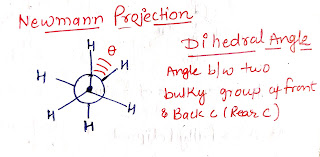
Conformation of Ethane :—
[CH3 - CH3]
Conformation of Propane :-
CH3 - CH2 - CH3 ये Identical है, लेकिन अलग - अलग होंगे तो ये बताना होगा कि किस C के Along Rotate होगा।
Conformation Of Butane :-
Stability Order :-
Anti Staggered > Gawche > Partially > Fully Eclipsed.
Conformation are stable due to
1. Torsional Strain
2. Steric Strain (Vander waal Strain)
Torsional Strain :-
Is due to Dihedral Angle.
Is due to Bond - Pair - Bondpair repultison.
Less Dihedral angle more will be the Torsional Strain.
Vander Waal Strain :-
Due to Repulsion of bulky group.
** Nett Repulsive Energy for Diss. Eclipse & Gawche Form. **
(1) H - H are Eclipsed to each other then nett repulsive energy is 1Kcal/mol.
(2) If CH3 - CH3 are Gouche To each other then nett repulsive energy is 0.9Kcal/mol.
(3) If CH3 - H are Eclipsed to each other then nett repulsive energy is 1.5Kcal.
(4) CH3 - CH3 are Eclipsed to each other then nett repulsive energy is 4Kcal.
Higher the Repulsive Energy less will be Stability.









0 Comments