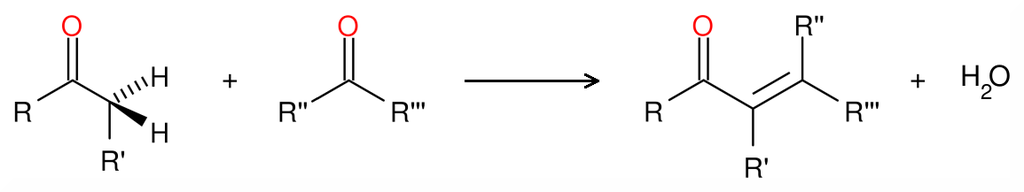
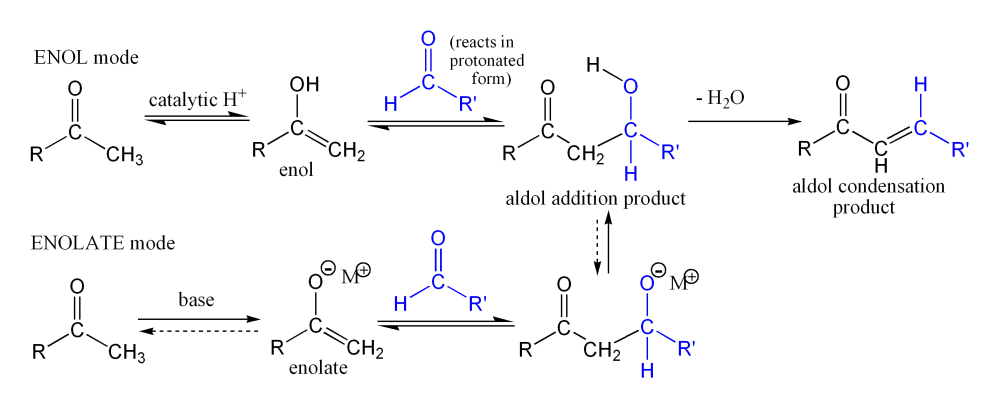
An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.
The reaction between an aldehyde/ketone and an aromatic carbonyl compound lacking an alpha-hydrogen (cross aldol condensation) is called the Claisen-Schmidt condensation. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this topic in 1880 and 1881. An example is the synthesis of dibenzylideneacetone. Quantitative yields in Claisen-Schmidt reactions have been reported in the absence of solvent using sodium hydroxide as the base and plus benzaldehydes.
Mechanism
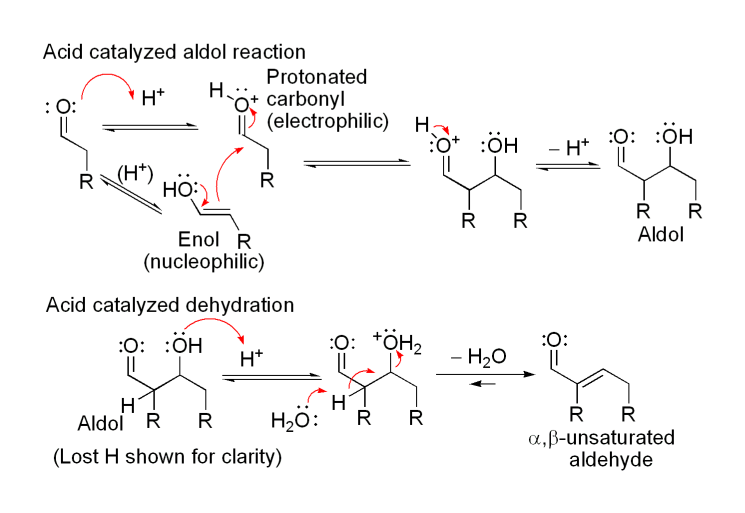
The first part of this reaction is an aldol reaction, the second part a dehydration—an elimination reaction (Involves removal of a water molecule or an alcohol molecule). Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid-catalyzed enol mechanism. Depending on the nature of the desired product, the aldol condensation may be carried out under two broad types of conditions: kinetic control or thermodynamic control.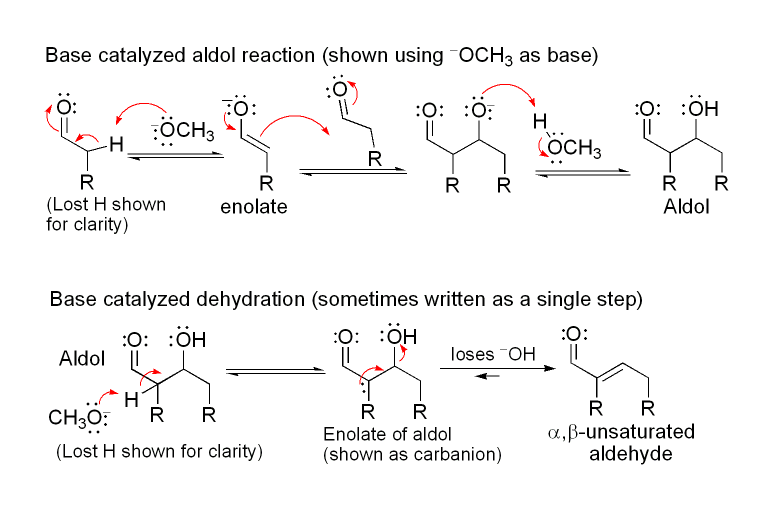
Condensation Types
It is important to distinguish the aldol condensation from other addition reactions of carbonyl compounds.- When the base is an amine and the active hydrogen compound is sufficiently activated the reaction is called a Knoevenagel condensation.
- In a Perkin reaction the aldehyde is aromatic and the enolate generated from an anhydride.
- A Claisen condensation involves two ester compounds.
- A Dieckmann condensation involves two ester groups in the same molecule and yields a cyclic molecule
- A Henry reaction involves an aldehyde and an aliphatic nitro compound.
- A Robinson annulation involves a α,β-unsaturated ketone and a carbonyl group, which first engage in a Michael reaction prior to the aldol condensation.
- In the Guerbet reaction, an aldehyde, formed in situ from an alcohol, self-condenses to the dimerized alcohol.
- In the Japp–Maitland condensation water is removed not by an elimination reaction but by a nucleophilic displacement
Aldol condensations are important in organic synthesis, because they
provide a good way to form carbon–carbon bonds. For example, the
Robinson annulation reaction sequence features an aldol condensation;
the Wieland-Miescher ketone product is an important starting material
for many organic syntheses. Aldol condensations are also commonly
discussed in university level organic chemistry classes as a good
bond-forming reaction that demonstrates important reaction mechanisms.
In its usual form, it involves the nucleophilic addition of a ketone
enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.

The reaction between an aldehyde/ketone and an aromatic carbonyl compound lacking an alpha-hydrogen (cross aldol condensation) is called the Claisen-Schmidt condensation. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this topic in 1880 and 1881. An example is the synthesis of dibenzylideneacetone. Quantitative yields in Claisen-Schmidt reactions have been reported in the absence of solvent using sodium hydroxide as the base and plus benzaldehydes.


Mechanism
The first part of this reaction is an aldol reaction, the second part a dehydration—an elimination reaction (Involves removal of a water molecule or an alcohol molecule). Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid-catalyzed enol mechanism. Depending on the nature of the desired product, the aldol condensation may be carried out under two broad types of conditions: kinetic control or thermodynamic control.Condensation Types
It is important to distinguish the aldol condensation from other addition reactions of carbonyl compounds.- When the base is an amine and the active hydrogen compound is sufficiently activated the reaction is called a Knoevenagel condensation.
- In a Perkin reaction the aldehyde is aromatic and the enolate generated from an anhydride.
- A Claisen condensation involves two ester compounds.
- A Dieckmann condensation involves two ester groups in the same molecule and yields a cyclic molecule
- A Henry reaction involves an aldehyde and an aliphatic nitro compound.
- A Robinson annulation involves a α,β-unsaturated ketone and a carbonyl group, which first engage in a Michael reaction prior to the aldol condensation.
- In the Guerbet reaction, an aldehyde, formed in situ from an alcohol, self-condenses to the dimerized alcohol.
- In the Japp–Maitland condensation water is removed not by an elimination reaction but by a nucleophilic displacement




0 Comments